Atlases
The Harvard Tissue Atlas (HTA) gathers image and -omic datasets into high-resolution molecular maps. Our atlases provide precise molecular data on cell types, states, and interactions in a preserved 3D environment, shedding light on the complex interactions between cellular and acellular structures in normal and diseased tissue. These data enable a deeper understanding of how diseases start and progress to improve how diseases are diagnosed and managed.
The Harvard Tissue Atlas datasets provide a foundation for future advances in precision medicine, such as early cancer detection, AI/ML predictive models, and disease stratification for clinical trials.
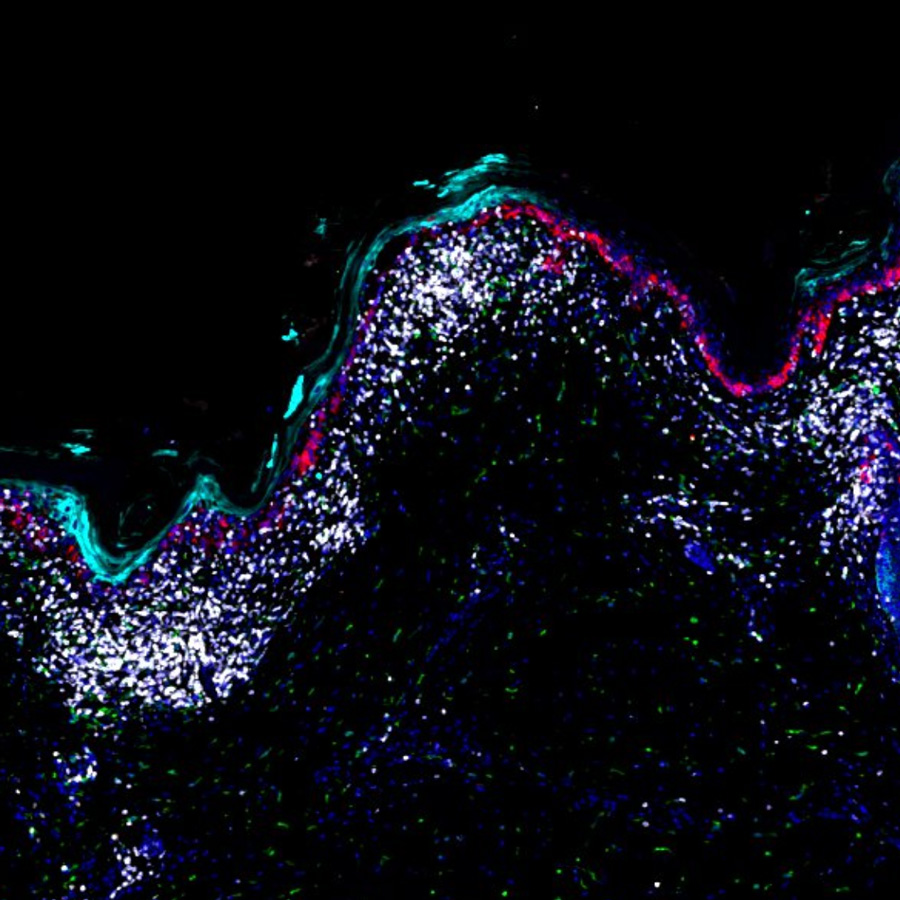
Melanoma Pre-Cancer and Progression Atlas
Melanoma is a highly immunogenic type of cancer treatable with minor surgery when localized to superficial layers of the skin but potentially lethal when it invades deep into the dermis and metastasizes. The Melanoma Pre-Cancer and Progression Atlas aims to identify the earliest molecular changes in pre-cancer and determine the sequence of events that ultimately leads to disseminated disease. This work is a component of the National Cancer Institute Human Tumor Atlas Network (HTAN)(a Cancer Moonshot Initiative) and Cancer Systems Biology Program.
Funded By:
NCI Human Tumor Atlas Network U2C-CA233262, NCI U54-CA225088, and the Ludwig Cancer Research Foundation
Explore Atlas
BRCA-positive Breast Cancer Atlas
The BRAC1/2 Cancer Atlas involves leading cancer centers across the US focused on collecting and analyzing diverse genomic and imaging on BRAC1/2 breast and ovarian cancers. The goal of the effort is to understand pre-cancer states, develop new diagnostics that detect cancer before it spreads, and improve disease management and prevention strategies.
Funded By:
NCI U54 CA225088 and The Gray Foundation
Explore Atlas
Colorectal Cancer Atlas
The Harvard Tissue Atlas for Colorectal Cancer aims to advance our understanding of the complex spatial interactions that contribute to colorectal cancer. Colorectal cancers are complex assemblies of tumor, immune, and stromal cells that invade adjacent tissue and spread to distant sites. We use highly multiplexed tissue imaging, spatial statistics, and machine learning to identify cell types and states underlying morphological features of known diagnostic and prognostic significance in colorectal cancer. This work is a component of the Human Tumor Atlas Network.
Funded By:
NCI Human Tumor Atlas Network U2C-CA233262, NCI U54-CA225088, and the Ludwig Cancer Research Foundation
Explore Atlas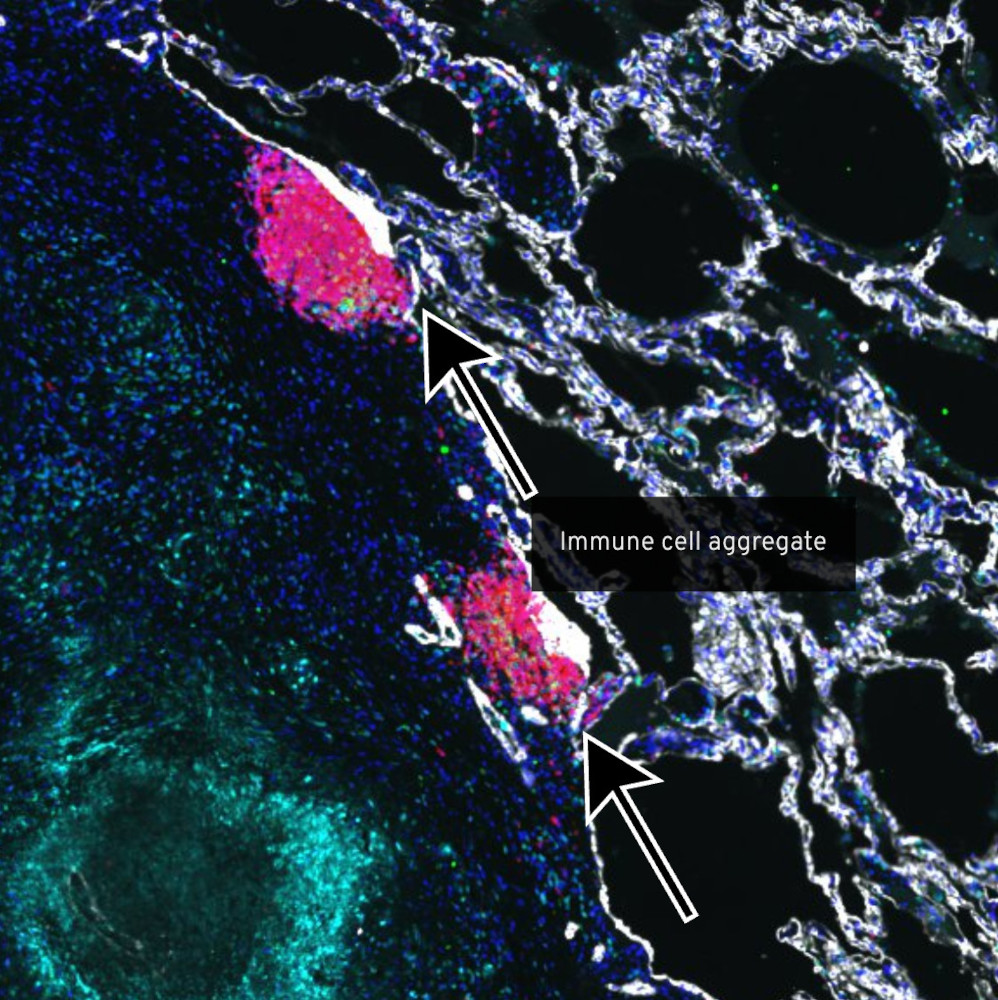
Tuberculosis Granuloma Atlas
The Tuberculosis Granuloma Atlas aims to transform our understanding of TB granulomas by using spatially resolved profiling methods that reveal bacterial, lung and immune cell organization, states, and communication within granulomas of different types.
Funded By:
Bill and Melinda Gates Foundation grant INV-027106
Explore Atlas
Rare Cancer Atlas
While all cancer diagnoses raise concerns and difficult questions for patients, their families, and their health care providers, rare cancers present additional challenges. Access to tumors and relevant samples for study is more limited, and commercial interest is lower because of decreased market size. The Bertarelli Rare Cancer Initiative supports advances in the detection, diagnosis, treatment, and prevention of rare cancers. Our vision is to build a world-class, highly interactive and collaborative hub for rare cancer research, clinical care advancement, advocacy and training, building upon the extensive and vibrant clinical and research communities across Harvard Medical School. Our long-term goal is to become an innovative world-wide initiative.
Funded By:
The Bertarelli Rare Cancer Initiative
Explore Atlas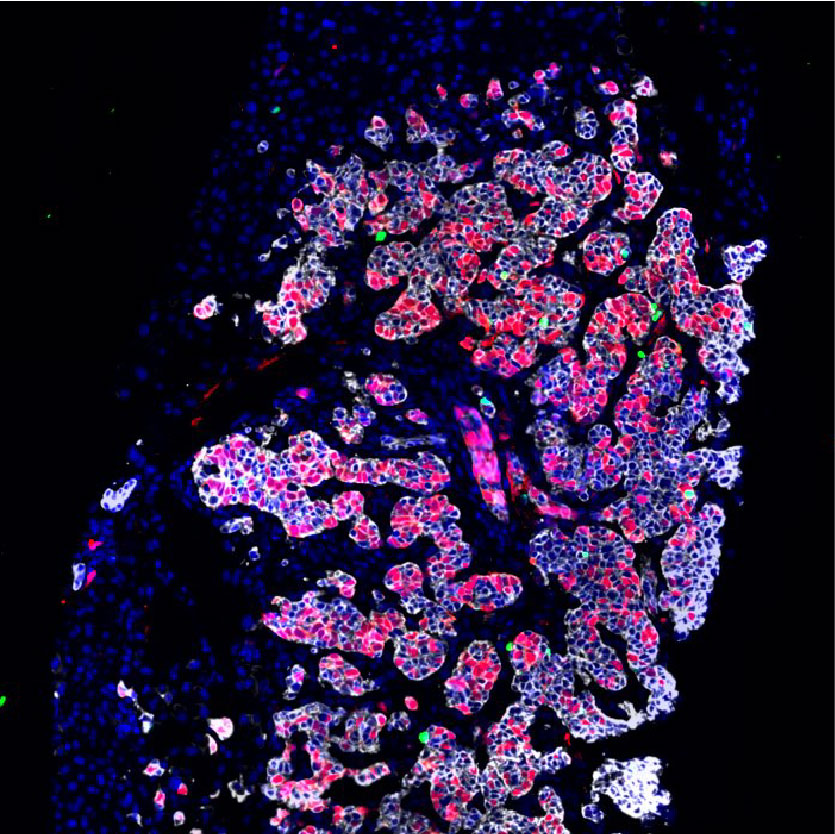
Breast Cancer Atlas
The Breast Cancer Atlas involves leading cancer centers across the US focused on collecting and analyzing diverse genomic and imaging on breast and ovarian cancers. The goal of the effort is to understand pre-cancer states, develop new diagnostics that detect cancer before it spreads, and improve disease management and prevention strategies. For research specifically on BRCA-positive breast cancer, visit the Gray BRCA Pre-cancer Atlas.
Funded By:
NCI U54 CA225088, The Gray Foundation, Ludwig Cancer Research
Explore Atlas
David Liposarcoma Research Initiative
Liposarcomas originate from precursors of fat cells are one of 250 types of sarcoma and are relatively rare, but they have few effective treatments. The David Liposarcoma Research Collaboration is led by DFCI Investigator George Demetri and aims to transform the treatment of this rare, underfunded, and understudied disease. As part of this effort, the LSP is collecting highly multiplexed images of liposarcomas before and after treatment.
Funded By:
David Liposarcoma Research Initiative
Explore Atlas