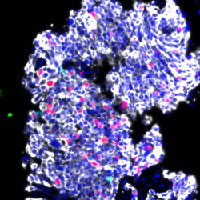
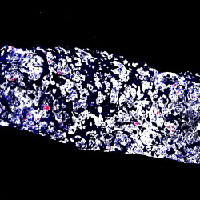
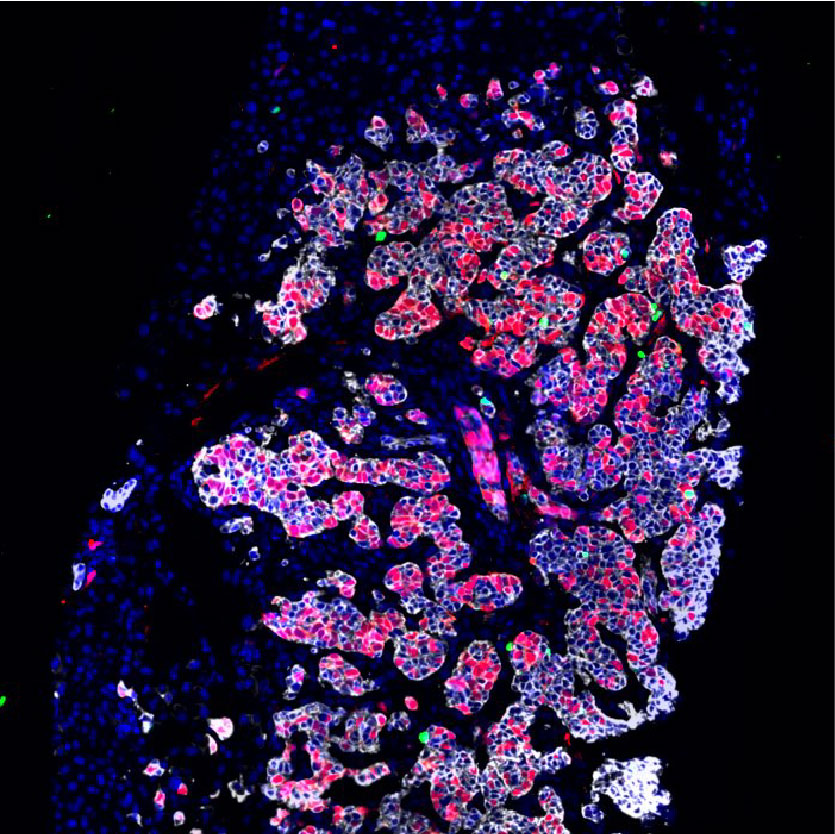
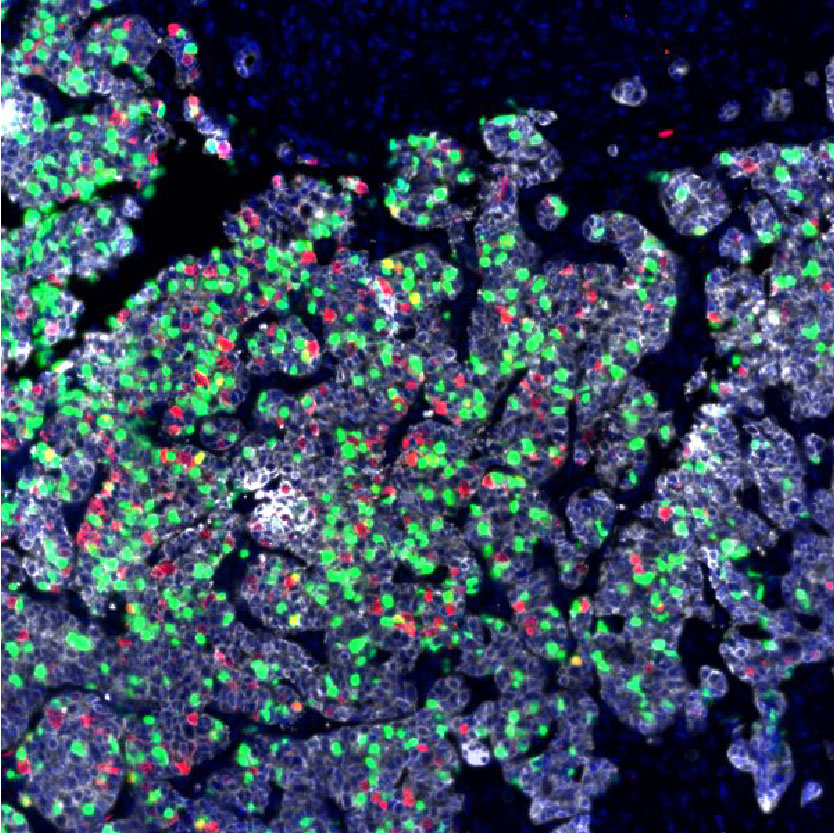
Clinical efficacy and molecular response correlates of the Wee1 inhibitor adavosertib with cisplatin in metastatic triple-negative breast cancer
Keenan T., Li T., Vallius T., Guerriero J.L., Tayob N., Kochupurakkal B., Davis J., Pastorello R., Tahara R.K., Anderson L., Conway J., He M.X., Shannon E., Godin R.E., Sorger P.K., D’Andrea A., Overmoyer B., Winer E.P., Mittendorf E.A., Van Allen E., Shapiro G.I., Tolaney S.M.
Clinical Cancer Research, 2(1), 66-82. PMID: 33257427
Selective inhibition of the negative cell cycle regulator WEE1 may enhance the efficacy of DNA-damaging agents by reducing DNA damage repair. These are multiplexed cyclic immunofluorescence on paired pre- and post-WEE1 inhibitor tumor biopsies, from the first phase II study assessing the efficacy of the WEE1 inhibitor adavosertib with cisplatin in metastatic triple-negative breast cancer (mTNBC). Among patients with mTNBC treated with 0-1 prior lines, adavosertib combined with cisplatin narrowly missed the prespecified ORR cutoff of > 30%. The finding of immune infiltrated tumors in patients with clinical benefit to therapy requires validation in future studies.
PublicationNarrated stories
Narrated stories use multi-step narrations and annotations to walk a viewer through key features of the data. Narrated stories distill the multidisciplinary knowledge encompassed by each dataset into a single product that grounds the scientific analyses in the underlying data and metadata. Click the Minerva story icon for an interactive view of the full-resolution images.